Tested to your
specifications
Each stability study is designed to your exact specifications. We offer accelerated and real-time testing, and can accommodate special requests, including freeze thaw studies and sterility integrity testing.
Get in touch to learn more
Give us a call:
1.800.209.4488
Monday to Friday
8:00AM–5:00PM PT
Why are stability studies important?
In the pharmaceutical and biopharmaceutical industries stability studies are performed to evaluate the stability of drug products under various environmental conditions. Once storage conditions have been defined, data from carefully designed stability studies are used to establish shelf life and to help determine retest dates for drug products. Although emphasis is usually placed on the stability of final products, the stability of reagents used in the manufacturing process is also an important consideration.
While the shelf life is readily available for off-the-shelf reagents, that's not the case for custom GMP formulations. For these, stringent stability studies need to be designed and performed to ensure that effective reagents are used throughout the manufacturing process. Because chemical degradation can occur over time, leading to lower concentrations of critical components or the accumulation of toxic substances, it is important to define the shelf lives of these critical intermediate reagents under specified conditions.
What is a stability study?
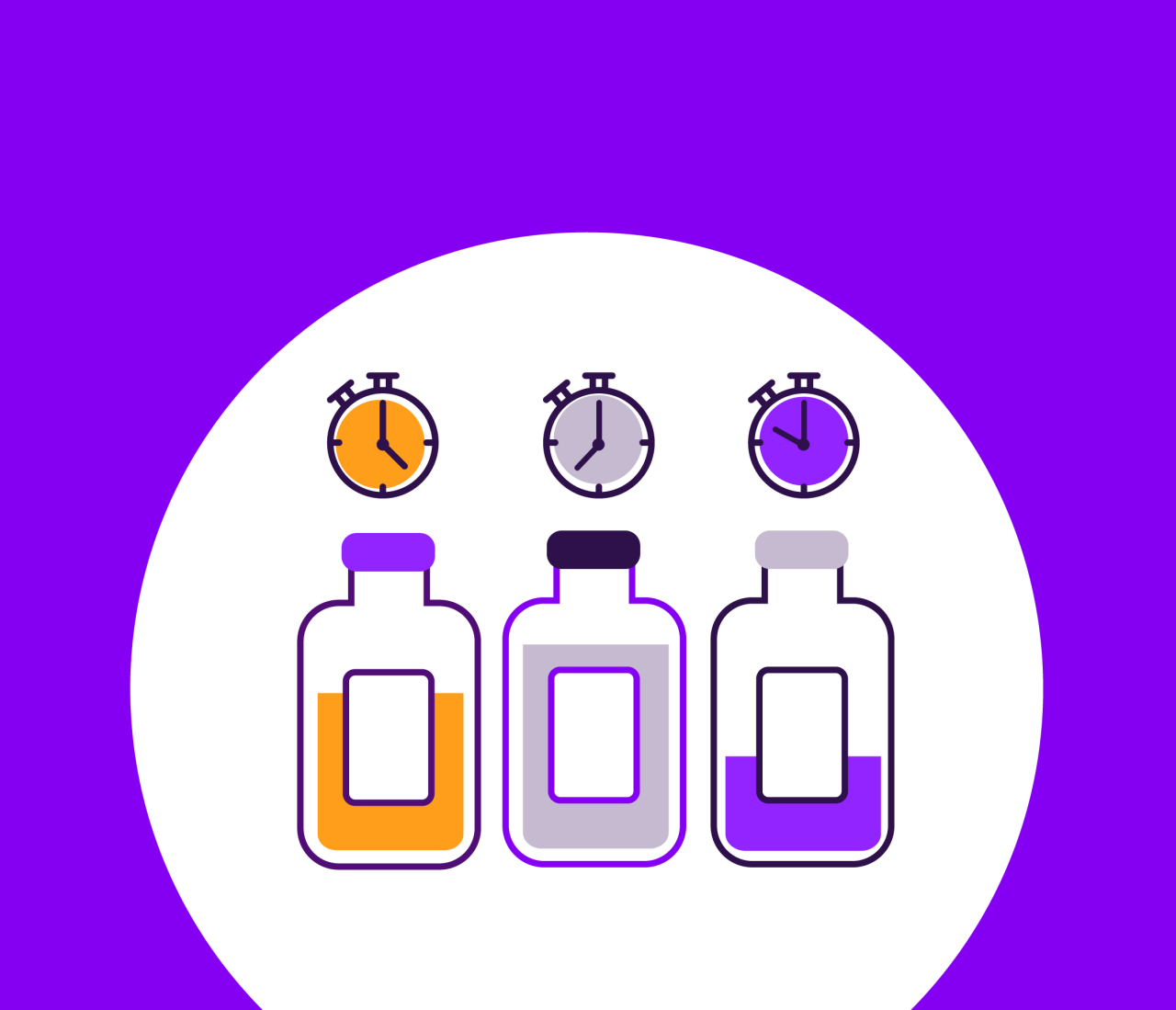
There are two main types of stability tests: accelerated and real-time (Figure 1). Accelerated testing is performed under increased stress by changing factors such as light, heat, and/or humidity. These conditions exacerbate the aging process to simulate long-term storage, providing a predictive view of what can be expected from real-time stability studies, which run concurrently.
However, extrapolating accelerated study results to “normal” storage conditions can be misleading and should be interpreted with caution (Ebrahim et al. Clin. Chem. 2021; 67: 684–688). Real-time stability testing follows the dictated storage conditions of the final product to allow the assessment of changes over extended periods of time. A typical study incorporates testing at 3, 6, 9, and 12 months during the first year. Subsequent testing occurs twice a year in the second year and once a year thereafter for a predetermined time.
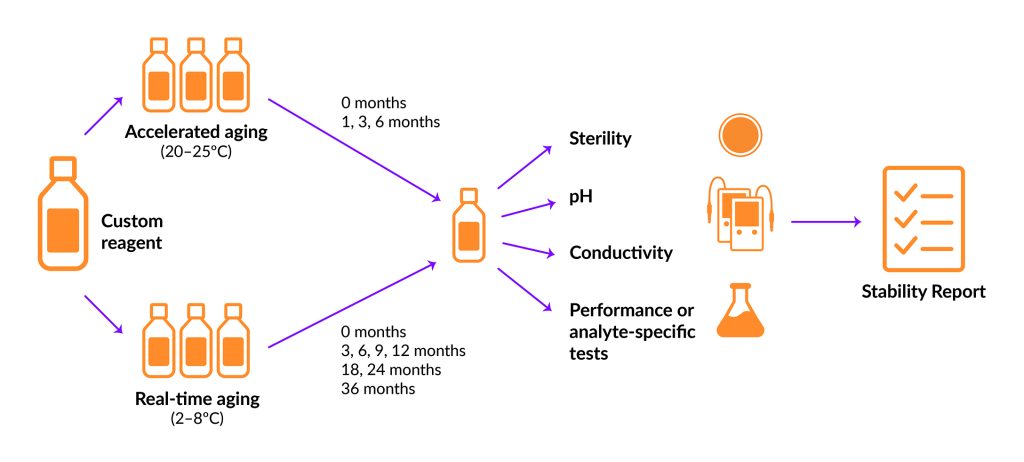
Figure 1. Comparison of accelerated and real-time stability studies.
What tests are used to establish stability?
Custom GMP reagents require carefully designed stability testing. Determining which tests are necessary will depend on the type of product and the product’s components and intended use. Key specifications that are included in the Certificate of Analysis (COA) also can inform the choice of tests.
Common metrics include pH, conductivity, density, osmolality, and appearance. The types of assays or measurements for concentration, performance, and activity will be tailored for specific components or applications.
| Common metrics | Additional metrics | Specialty metrics |
|---|---|---|
| pH | Sterility | Concentration |
| Conductivity | RNase | Performance |
| Density | DNase | |
| Osmolality | PCR | |
| Appearance | Endotoxin |
Understanding the scope of stability studies
The scope of a stability study will depend on the sample volume, the number of stability tests needed, and the duration of the study. Stability tests are typically done on samples that are in the final packaging format. For example, sample volumes and container types typically match those of the final product.
The testing plan and timepoints need to be determined and factored into the manufacturing process since the volume of reagent in the lots must account for the amount needed to complete the stability study. Given the number of lots, controls, technical replicates, and tests that are run at each time point, the number of individual tests can increase quickly.
We are equiped with a wide range of instruments for stability testing and our staff is trained to perform tests following standardized protocols. We take into account the planned study duration, alloting long-term storage facilities to store study samples under controlled environmental conditions.
How we can help
To help increase your team's efficiency we can both manufacture your custom formulations and design and perform a stability study for that product. We have the facilities, equipment, and expertise to ensure batch-to-batch consistency and perform customized quality control and stability testing. Our team of trained scientists and quality control experts can provide consultation on study design and regulatory requirements, and perform the selected studies.
When we perform stability studies on the reagents we manufacture, we use the release criteria for each lot as a true time-zero data point, meaning this data was obtained using standardized protocols and the same instruments that will be used throughout the studies. We also maintain retention samples to assist in troubleshooting at any point during the product's lifespan.
CASE STUDY
A stability study for a custom buffer that improves yields in gene therapy manufacturing
Say a gene therapy manufacturing company requests a custom buffer containing an optimized, critical component: magnesium chloride (MgCl2). We will need to ensure that the amount of MgCl2 in the buffer remains at a specific concentration over the life of the product because any changes can decrease the efficiency of viral production.
Once we manufacture the buffer, our scientists will perform experiments that confirm the concentration of the critical component (for example, by analytical titration and atomic absorption spectroscopy). This methodology is used to monitor MgCl2 concentrations during the stability studies. These particular buffer samples are then also tested for sterility, pH, and conductivity.
To summarize:
- Sterility testing (based on USP <71>) is typically included for reassurance since sterility of the unopened product should remain unchanged. Because this is primarily an assessment of the container closure system, some study plans include sterility testing once a year, instead of at every time point.
- The pH (based on USP <791>) and the conductivity (based on USP <645>) are used as standard, general measures of stability in biopharma settings. Changes could indicate degradation of components in the buffer.
- The MgCl2 concentration is monitored by following the USP monograph for magnesium chloride, which dictates testing methodology.
In keeping with a comprehensive stability study design, we include samples from multiple lots of buffer in accelerated and real-time testing. The accelerated study is shorter, and buffer samples from three lots are stored at a higher temperature (in this case, timepoints at 1, 3, and 6 months with samples stored at 25°C). In contrast, the real-time study is longer in duration, and samples from the same three lots are stored at recommended conditions (in this case, timepoints at 3, 6, 9, 12, 18, 24, and 36 months with samples stored at 4°C).
Table 1. Number of sample tests in a comprehensive stability study. Note that each sample is used to produce three technical replicates for each test.
Total numbers (not including controls) | Accelerated (3 lots, 3 technical replicates, 4 tests, 3 timepoints) | Real-time (3 lots, 3 technical replicates, 4 tests, 7 timepoints) |
|---|---|---|
Samples (lots x timepoints) | 9 | 21 |
Tests run at each timepoint (lots x replicates x tests) | 36 | 36 |
Tests run during the stability study (lots x replicates x tests x timepoints) | 108 | 252 |
The accelerated stability study supports the use of the new custom buffer during early research and development until the real-time data is available. The gene therapy manufacturer can determine the shelf life of the buffer under the specified storage conditions based on data from the real-time study.
Key takeaways
In the long term, investing in stability testing of custom reagents helps remove a potential variable in the complex workflow for development and manufacturing of novel biotherapeutics.
The optimal design (which includes the selection of tests and timepoints) of a stability study depends on the composition of the custom reagent and its intended use. Stability testing provides supporting evidence that helps ensure GMP products meet both internal and external requirements, and that contributes to the production of safe, pure, and effective consumer products.
Yes. We can perform stability studies on products from any vendor. If it's a product we also manufacture, we can integrate the stability study into the manufacturing process to increase efficiency, and eliminate variables: the same equipment will be used throughout, with every step of the process tailored to your quality control criteria.
It's ideal to start the process when you are getting ready to transition from RUO to GMP. We can help you plan for the transition and can build your stability study into the manufacturing of your first GMP lot.
Yes. Stability studies can be used both to assign a shelf life, or to confirm if it can be safely extended. If you have a product that's nearing it's expiration date, get in touch and we can pull the retention sample to begin the testing process.
Accelerated testing is performed under increased stress by changing factors such as light, heat, and/or humidity. These conditions exacerbate the aging process to simulate long-term storage, providing a predictive view of what can be expected from real-time stability studies, which run concurrently.
Real-time stability testing assesses changes over extended periods of time when following the recommended storage conditions for the final product. A typical study incorporates testing at 3, 6, 9, and 12 months during the first year. Subsequent testing occurs twice a year in the second year and once a year thereafter for a predetermined time.
Yes. We can perform tests for a variety of environmental factors including freeze thaw studies and sterility integrity testing. Get in touch to discuss any unique conditions you would like to test for and we can design a custom testing program.
Discover more resources