AAV2
The AEX Buffer Screening Kit is part of our line of
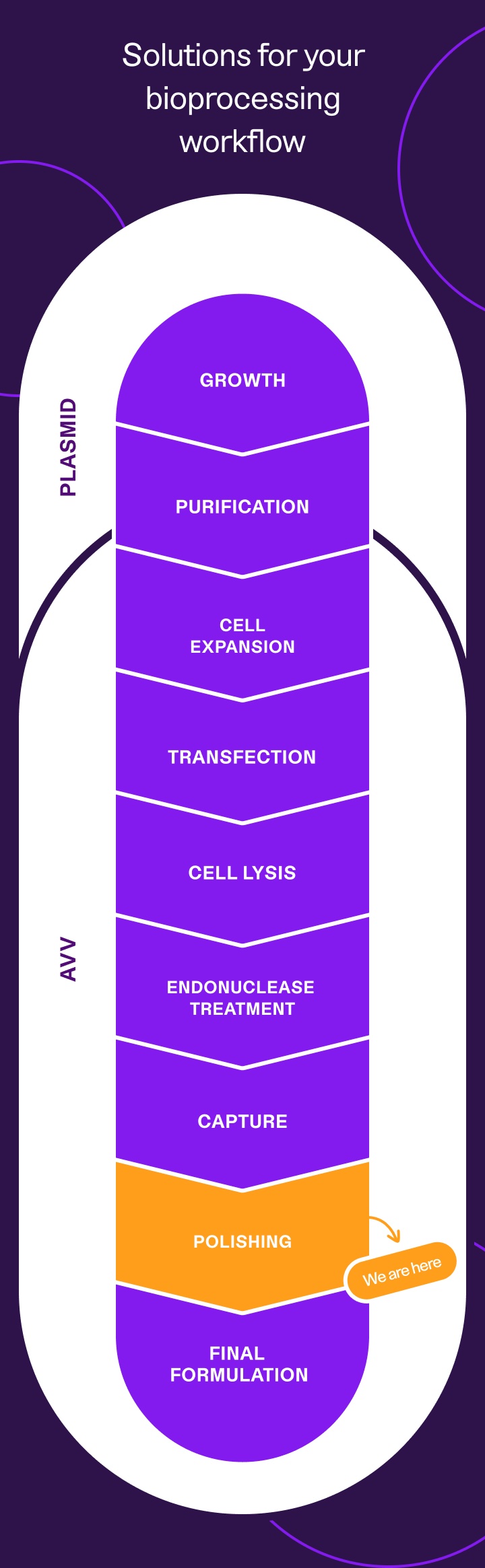
end-to-end reagents for every step of your AAV workflow
Keep scrolling to learn more about how our AEX Buffer Screening Kit can save you months at the polishing step,
or explore our AAV workflow to find tailored solutions for every step of your AAV process.

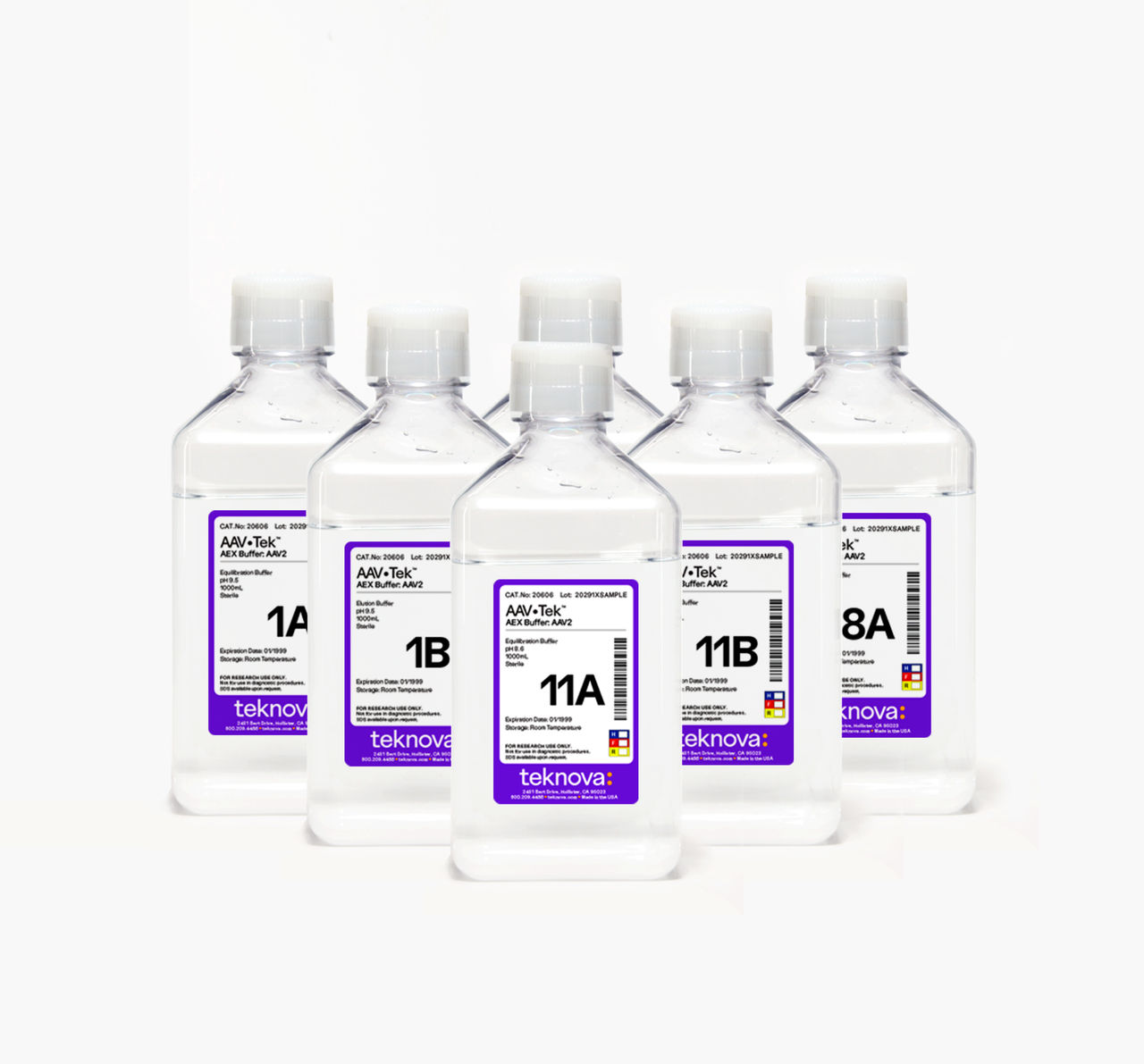
AAV•Tek AEX Buffer Screening Kit
Now available for
AAV2, AAV6, and AAV8
Through extensive screening, we created a first-of-its-kind kit that can help you identify the ideal buffer formulation for the separation of empty and full capsids while maintaining high yields, saving months in your process development at the polishing step.
Each serotype-specific kit contains paired sets of optimized equilibration and elution buffers in 1 L bottles. These discrete formulations have been proven to be effective across multiple upstream and purification platforms.
Explore the products in the kits
AAV6
AAV8
AAV polishing,
simplified
With decades of experience manufacturing custom buffers and expertise in the AAV bioproduction process, we can help simplify your AAV workflow.

- AAV2
- AAV6
- AAV8
Different DNA sequences that are packaged within the viral capsid may impact the pI of the overall construct. Therefore, the optimized buffer formulations were validated against multiple sequences, as shown in these figures.

Initial work implementing the DOE was performed using monolith technology due to the high flow rates associated with these columns. After identification of the optimal recipe, however, the formulation was validated across multiple other platforms, including two commonly used AEX resins shown in the figure below.

To ensure the kit is agnostic of the upstream process used (for instance, cell line, media choice, transfection and harvest conditions) and does not affect the ability to successfully utilize the buffer set, multiple processes were implemented and tested using the optimal buffer formulations.

Variations to the downstream processes, such as TFF, affinity, and CEX, may affect the final AEX polishing step. To probe this concern, multiple downstream processes were implemented to verify the applicability of the optimized buffer formulations.

The figure below demonstrates the utility of the kit for various chromatographic modalities. The buffer screening is shown using a linear gradient (left) and then finalized using an isocratic hold (right) to facilitate scale-up requirements.

Different DNA sequences that are packaged within the viral capsid may impact the pI of the overall construct. Therefore, the optimized buffer formulations were validated against multiple sequences, as shown in these figures.

Initial work implementing the DOE was performed using monolith technology due to the high flow rates associated with these columns. After identification of the optimal recipe, however, the formulation was validated across multiple other platforms, including two commonly used AEX resins shown in the figure below.

To ensure the kit is agnostic of the upstream process used (for instance, cell line, media choice, transfection and harvest conditions) and does not affect the ability to successfully utilize the buffer set, multiple processes were implemented and tested using the optimal buffer formulations.

Variations to the downstream processes, such as TFF, affinity, and CEX, may affect the final AEX polishing step. To probe this concern, multiple downstream processes were implemented to verify the applicability of the optimized buffer formulations.

The figure below demonstrates the utility of the kit for various chromatographic modalities. The buffer screening is shown using a linear gradient (left) and then finalized using an isocratic hold (right) to facilitate scale-up requirements.

Different DNA sequences that are packaged within the viral capsid may impact the pI of the overall construct. Therefore, the optimized buffer formulations were validated against multiple sequences, as shown in these figures.

Initial work implementing the DOE was performed using monolith technology due to the high flow rates associated with these columns. After identification of the optimal recipe, however, the formulation was validated across multiple other platforms, including two commonly used AEX resins shown in the figure below.

To ensure the kit is agnostic of the upstream process used (for instance, cell line, media choice, transfection and harvest conditions) and does not affect the ability to successfully utilize the buffer set, multiple processes were implemented and tested using the optimal buffer formulations.

Variations to the downstream processes, such as TFF, affinity, and CEX, may affect the final AEX polishing step. To probe this concern, multiple downstream processes were implemented to verify the applicability of the optimized buffer formulations.

The figure below demonstrates the utility of the kit for various chromatographic modalities. The buffer screening is shown using a linear gradient (left) and then finalized using an isocratic hold (right) to facilitate scale-up requirements.

TAKEAWAY
Product guide
Here's a quick reference sheet with all the key information
about our AEX Buffer Screening Kits for AAV2, AAV6, and AAV8.
Frequently asked questions
The AAV•Tek AEX Buffer Screening Kit helps with a critical step in the AAV downstream purification process: the anion-exchange (AEX) polishing, or empty capsid removal, step. The kit includes a selection of AAV serotype-specific equilibration and elution buffers that have been optimized to help you save weeks to months in defining the right AEX buffers for your process.
The buffers have been developed by monitoring four different critical quality attributes (CQAs): purity, recovery, infectivity, and peak separation. Depending on your end application, some buffers may be more suitable based on the CQAs for your intended use. For each serotype-specific kit we’ve selected the optimal number of buffers to help you quickly find the best buffer for your application.
We currently offer AAV•Tek AEX Buffer Screening Kits for 2 serotypes: AAV2 and AAV8. We are working on solutions for further AAV serotypes, including AAV5, AAV6, and AAV9. If you are working with other serotypes and are interested in the kit, get in touch and let us know your serotype to help us prioritize our future developments.
The AAV•Tek AEX Buffer Screening Kits have been optimized for standard AAV serotypes containing a selection of genes of interest. Depending on the extent of modifications from the standard serotype, the buffers in the kits may or may not be suitable. Contact our experts for a consultation. We may be able to run a DOE for your specific serotype.
Each kit is optimized for a specific serotype, and the number of equilibration and elution buffers provided in the kits varies based on the requirements of that serotype. The AAV2 kit includes 7 pairs of buffers (14 bottles total) and the AAV8 kit has 6 (12 bottles total). Each buffer is supplied in a 1 L quantity. Once you have found your formulation, we can produce it in larger quantities to support your scale up.
The equilibration and elution buffers included in the kits are designed to work in specific pairings based on their compositions. We do not recommend mixing the number associated with the “A” and “B” components.
We recommend starting with the complete kit to define the best buffer set for your viral capsid. However, individual bottles of equilibration and elution buffers can be purchased separately for additional experiments and scale up.
All buffers included in the screening kits contain raw materials that are compatible with scale up and GMP manufacturing. Once you have identified the equilibration and elution buffers that work best for your process, we can help make further customizations as needed and manufacture your unique formulation in larger quantities, up to 200 L bags.
Our kits are manufactured to the same rigorous standards as all our products. All the buffers in the kit are endotoxin tested (<0.5 EU/mL) and have tight pH specifications (± 0.05). We also test and report conductivity, osmolality, density, and sterility on the Certificate of Analysis.
Yes. The kit is developed for use in a linear gradient modality to identify the best buffer for your needs, however, each buffer can be adapted for use in isocratic or step elution modalities if that is necessary for your manufacturing process. We’ll partner with you to determine the optimal formulation from the screening kit for the next steps in your scale up process. Once you've identified the step elution or isocratic hold buffers that work best for your process, we can manufacture your capsid-specific buffers in the quantities you need.
We screened hundreds of parameters to develop the serotype-specific buffers included in the kits. While the formulations of the specific buffers in our AEX Buffer Screening Kits are proprietary to Teknova, once you are ready to scale up and use GMP versions of your ideal buffers, we will share the formulations with you under our non-disclosure supply agreement.
If the pre-AEX step eluate has a conductivity below 5.5 mS/cm?
Dilute the pre-AEX step eluate with 10-fold dilution of the AEX A (Equilibration) Buffer, verify that the conductivity is no more than (NMT) 5.5 mS/cm, and adjust/verify that the pH is ± 0.1 of the AEX A (Equilibration) Buffer. Remeasure conductivity if a pH adjustment was required and verify that it is NMT 5.5 mS/cm.
If the pre-AEX step eluate has a conductivity greater than 5.5 mS/cm?
Option 1. Dilute the pre-AEX step eluate with 17 to 20-fold or possibly more (e.g., 25-fold) with AEX A (Equilibration) Buffer, verify that the conductivity is no more than (NMT) 5.5 mS/cm, and adjust/verify that the pH is ± 0.1 of the AEX A (Equilibration) Buffer. Remeasure conductivity if a pH adjustment was required and verify that it is NMT 5.5 mS/cm.
Option 2. If Option 1 doesn’t reduce the conductivity enough, adjust conductivity with a non-salt containing buffer with a pH ± 0.1 of the AEX A (Equilibration) Buffer, verify that the conductivity is no more than (NMT) 5.5 mS/cm, and adjust/verify that the pH is ± 0.1 of the AEX A (Equilibration) Buffer. Remeasure conductivity if a pH adjustment was required and verify that it is NMT 5.5 mS/cm.
Find out what’s possible
Talk to our consultants today to discover custom solutions that can help you achieve your goals.